Doctors and nurses want B.C. to rethink 'extremely alarming' delay to 2nd COVID-19 vaccine dose
2 letters obtained by CBC show concerns about lack of evidence and informed consent
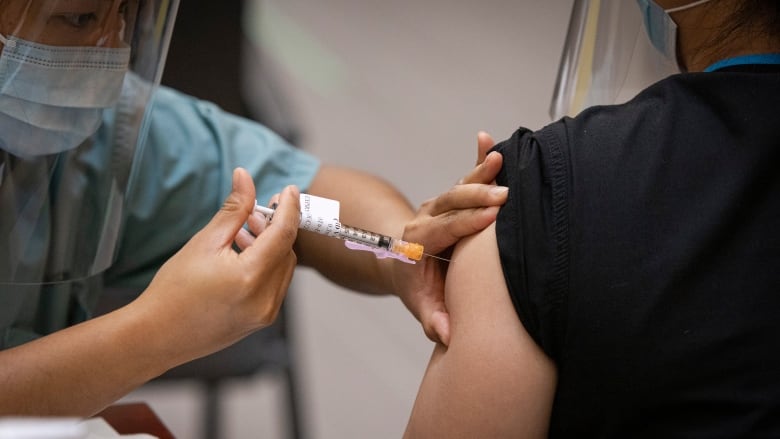
Two separate groups of B.C. doctors and nurses are calling for the province to reverse course on a decision to delay second doses of the COVID-19 vaccine, arguing the move is unscientific, unsafe and unethical.
Their concerns are laid out in two letters obtained by CBC News one from the Doctors of B.C.'s section of emergency medicine and the other signed by more than 100 nurses from across B.C.
The Doctors of B.C. letter calls for help from its leadership toraise the issue with provincial officials.
"We understand the rationale for this decision; however, there are several reasons why this decision by public health is extremely alarming, unethical and a violation of the rights to informed consent," the letter says.
On Dec. 24, Provincial Health Officer Dr. Bonnie Henry revealed that B.C. will delay second doses of COVID-19 vaccines to up to 35 days after the first shot.
That's despite federal authorizations and guidelines that say the Pfizer-BioNTech shots should be spaced out by 21 to 28 days and the Moderna shots by 28 days. The recommended timing for the doses comes directly from the manufacturers, based on their clinical trials.
Researchers have found the Pfizer-BioNTech vaccine to be just 52 per cent effectiveafter one dose;however, Public Health England arguesits review of the data suggests efficacy rises to about 89 per cent by 15 days after the first shot.The Moderna shot is estimated to be 80.2 per cent effective after a single dose.
The doctors' and nurses' letters raise similar concerns about the lack of data on the efficacy of the vaccines if second doses are delayed.
"By not following the advocated guideline, B.C. has elected to unroll the vaccine in a fashion that is not supported by the existing evidence and could be considered 'experimental,'" the doctors' letter says.
A spokesperson for the Doctors of B.C. said representatives are meeting with government officials, including Henry, to discuss the doctors' concerns about vaccine rollout.
During Thursday's news briefing, Henry defended the decision to delay second doses.
"We did an ethical review, we looked at the data, we looked at the modelling of how many people we could best protect and we looked at the operational issues of how much vaccine we're expecting," she said.
"Absolutely, the science bears out that this is a reasonable approach that maximizes our ability to protect more people during this, the most infectious periodwhere we have the highest risk of transmission."
Delayed doses an 'experiment,' letters say
Both letters also raise ethical concerns about informed consent, arguing that those who received a first dose before Dec. 24 understood they would receive the second on the authorized schedule.
The nurses' letter to Henry and Health Minister Adrian Dix says, "Individuals were explicitly informed by those who were administering the vaccination that a second dose would be required within 21 days for the Pfizer vaccine.
"The decision to announce a delayed vaccination schedule after doses have begun to be administered is in itself an experiment that we unknowingly became a part of and that we did not consent to."
But Henry disputed that characterization Thursday, saying, "What people consented to was a two-dose series."

B.C. Nurses Union president Christine Sorensen, meanwhile, says she passed alongnurses' concernsabout the delayed second-dose to the Ministry of Health earlier this week.
"It's interesting because we follow the other guidelines from the manufacturer around storage and handling of this vaccine," said Sorensen, "But now we're going to deviate?"
World Health Organization weighs in
On Friday, World Health Organization experts advised that the interval between the two doses of the Pfizer-BioNTech vaccinecan be extended to up to six weeks.
The advice, which comes from WHO'sStrategic Advisory Group of Experts on immunization, maintains that an interval of 21 to 28 days between doses is recommended, but notes"a number of countries face exceptional circumstances of vaccine supply constraints combined with a high disease burden," and suggests that window could expand to 42 days in order to broaden coverage.
Sorensen, however, says the recommendationis intended for "areas ofextremely high rates of COVID" such as the United Statesand United Kingdom.
Health Canada currently recommends the doses be given "as close as possible to the authorized dosing regimen," according to an email from a spokesperson.
However, theNational Advisory Committee on Immunization is currently looking into the scientific and ethical evidence on delaying second doses so as many people as possible can receive the shot, and an update is expected soon.
The U.S. Food and Drug Administration, on the other hand, has come out strongly against delaying second doses, calling the idea "premature and not rooted solidly in the available evidence."
In a statement released Monday, the American body said there is a significant risk to public health in changing the vaccine schedule.
"We know that some of these discussions about changing the dosing schedule or dose are based on a belief that changing the dose or dosing schedule can help get more vaccine to the public faster," the statement reads.
"However, making such changes that are not supported by adequate scientific evidence may ultimately be counterproductive to public health."
With files from Ethan Sawyer












_(720p).jpg)


 OFFICIAL HD MUSIC VIDEO.jpg)
.jpg)



























































































