Omicron-specific COVID-19 vaccine now available to some New Brunswickers
Moderna's so-called bivalent vaccine is being offered to high-risk groups first
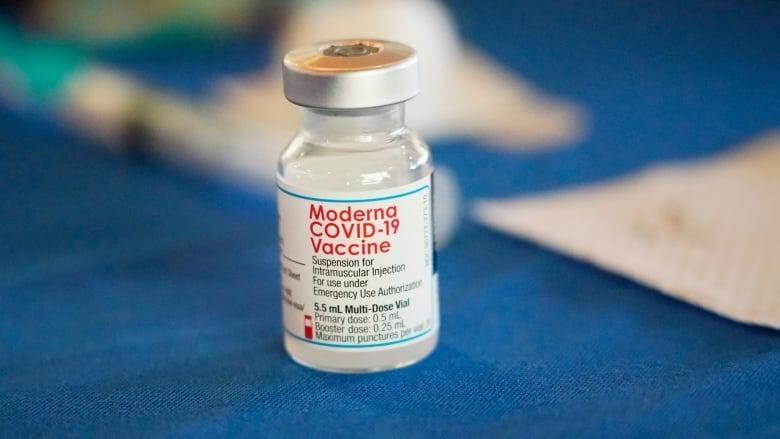
Some New Brunswickersnow have access to thenewCOVID-19 vaccine thattargets the Omicron variant BA.1, as well as the original coronavirus.
People 50 and older, those aged12 to 17 who are immunocompromised or have a high-risk medical condition, and those aged 18 and older who live in a First Nations community areeligible for the so-called bivalent vaccine,as long as five months have passed since their last vaccine dose or a COVID-19 infection, the Department of Healthannounced Wednesday.
"Eligible New Brunswickers should be able to book an appointment for a Moderna Spikevax Bivalent Original/Omicron BA.1 booster vaccine at some participating pharmacies as early as today, with more pharmacies coming online as we progress through the week," spokesperson Adam Bowie told CBC News in an email.
The regional health authorities may also continue to offer some vaccination clinics where access is limited in certain locations around the province, based on community demand, he said.
Until now, only monovalent vaccines,protecting against only one strain of the virus, have been available in the province.

Dr. Arifur Rahman,the province's acting deputy chief medical officer of health, said it's important for people to stay up-to-date with boosters, even if they're not eligible for the bivalent vaccine yet.
"We plan to expand eligibility criteria as more bivalent vaccines become available, but individuals eligible for a fall booster dose should not delay getting their planned COVID-19 vaccination in anticipation of a bivalent vaccine," he said in a statement.
"Vaccines are our best defence against severe outcomes."
The Omicron variant BA.1 emerged late last year and drove thelargest wave of infection and hospitalizationin the pandemic.
Of the most recentrandom samples sent for sequencing in the province, 98 per cent were the highly transmissible Omicron subvariantBA.5,one per cent were the Omicron subvariant BA.4, and the other one per cent were Omicron subvariant BA.2.
Most vulnerable first
In Quebec,the bivalent vaccine has been offered to all adults, as well assome teens and children, for aweek.
Health Canada approvedthe bivalent on Sept. 1.
Jake Reid, executive director of the New Brunswick Pharmacists' Association, expects the province will open upeligibility within weeks.
"One of the things that we were told as pharmacists is that for the inventory that we have on hand, we want to make sure that we protect the most vulnerable. And so we're going to do that first and then we'll be able to offer it to everyone else afterwards," he said.
Some First Nation communities will offer clinics for their community members "in the near future," the Department of Health spokesperson said, and residents in long-term care facilities will also be eligible to receive a bivalent shot, along with their influenza vaccine, beginning in October.

Vaccination clinics at pharmacies willrun asinventory comes in, according to Reid.
"The inventory for bivalent COVID vaccines is going to be, in some ways, replacing what we have right now. So I anticipate that the pharmacies who are right now part of the COVID vaccination program would continue and that they will be able to offer bivalentas soon as theyhave it."
It's difficult togauge interest in this new formulation,Reid said.
"There's definitely a vocal part of the population who are excited by this," he said. "They understand that this is a new version of the vaccine, that it's going to specifically target the Omicron variant, which we know spreads much faster through the population."
But overall uptake of vaccines and boosters hasbeen trending down, Reid noted.
"Not as many people have been getting their boosters when they're due for them. Younger people aren't getting their vaccines when they should be sometimes."
As of Tuesday, 90.5 per cent of eligible New Brunswickers have received their first dose of a COVID-19 vaccine, 85.4 per cent have received their second dose, 53.6 per cent have received their third dose, also known as a first booster, and 20.9 per cent have received a fourth dose, or second booster, Rahman said.
The Department of Health did not respond to a request to provide a breakdown by ages.
Paxlovid announcement pending
Changes to the guidelines for the antiviral COVID-19 treatment drug Paxlovidare expected next week, according to the news release.
The Department of Health is still reviewing the eligibility criteria for the drug, currently only available to"people who are at high risk of serious illness."That includes people aged 80 or older and others who are "moderately to severely immunocompromised."
Paxlovid is approved for peoplewho have mild to moderate symptoms of the virus.
"The department is examining ways to improve access to the oral antiviral and will have more details to share next week," the release said.
Paxlovid reduced the risk of hospitalization or death by89 per cent in non-hospitalizedhigh-risk adults with COVID-19, compared to a placebo during its clinical trial, according to Pfizer.
The drug must be taken within five days of the onset of symptoms.
Afull course of treatment consists of 30 pills over five days.











_(720p).jpg)


 OFFICIAL HD MUSIC VIDEO.jpg)
.jpg)