2 young Canadian brothers, a life-threatening disease and the harsh reality of drug prices
Its not just in Canada. All over the world patients are left out as a bitter drug-price fight rages

This is an excerpt from Second Opinion, a weekly roundup of eclectic and under-the-radar health and medical science news emailed to subscribers every Saturday morning. If you haven't subscribed yet, you can do that by clicking here.
Two young brothers with cystic fibrosis (CF) each struggling with the same life-threatening disease. But only one of them is getting treated with a breakthrough drug.
Andre Larocque, 8, got lucky. He was picked for a clinical trial that gives him access to a new CF drug for free. It's called Symdeko.
His younger brother, Joshua, 6, might benefit from a similar version of the drug already on the market, called Orkambi. But he can't have it because it costs $250,000 a year.
That's the bleak reality at the heart of a family drama revealed by CBC's Go Public this week.
And it throws open the curtain on a problem that affects all Canadians.
There's lots of places around the world that have said, 'I'm sorry, but your price is unconscionably high.'- DiarmaidMcDonald, lead organizer, Just Treatment advocacy group
When a drug is overpriced even a rare drug it affects the entire health-care system, according to Steve Morgan, health economist and a professor at the School of Population and Public Health at the University of British Columbia.
"Canadians need to understand that when manufacturers are asking these prices they're essentially asking the health-care system to forgo other opportunities to provide patients with benefits from other technologies."
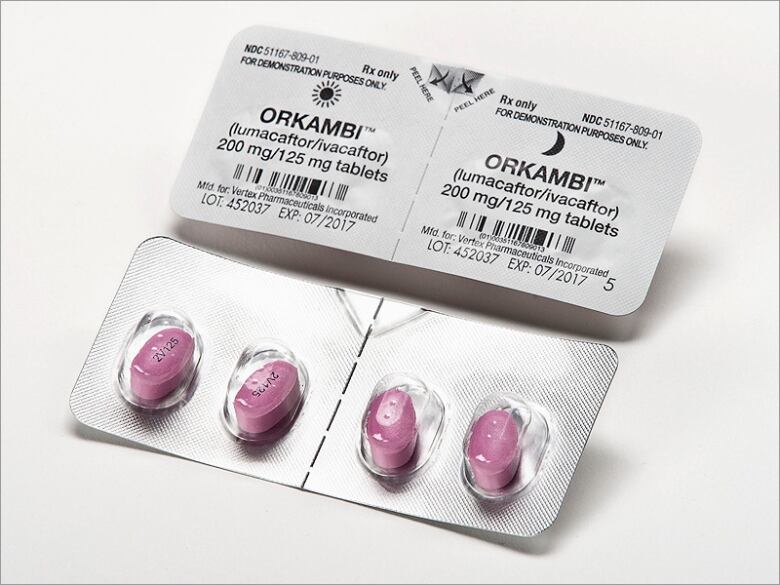
And the CF drugOrkambiis overpriced. That's according to cost-benefit analyses in Canada, the United Kingdom, and other jurisdictions, including New York State.
"There's lots of places around the world that have said, 'I'm sorry, but your price is unconscionably high,'" said Diarmaid McDonald, lead organizer of the U.K. drug advocacy group Just Treatment, which is campaigning to lower the price of Orkambi.
In Canada, all new drugs go through a health technology assessment by the Canadian Agency for Drugs, Technology and Health (CADTH). The agency then makes a non-binding recommendation to provinces about whether they should pay for the drug.
Twice the agency's experts have reviewed Orkambi, and twice they made the same decision recommending that provinces not pay for the drug at its current price.
At $170 for a single pill, Orkambi costs about $680 every day, according to the CADTH report. Added up, that's $249,000 per year more than the average Canadian earns in five years.
And while the company's clinical trial evidence showed some improvement in lung function and fewer lung infections for some patients, overall the committee decided that it wasn't clear how well the drug worked.
"In the end, our committee looking at the data thought it was too uncertain to recommend that this product be funded by the public system," said Trevor Richter, director of the CADTH Common Drug Review.
Vertex not submitting newest CF drug for review
Around the world, governments depend on cost-benefit assessments by agencies like CADTH to decide whether a public health system should pay for a drug.
And Vertex Pharmaceuticals, the maker of Orkambi, is not happy with the answers it's been getting.That's because other countries have also decided that Orkambi is too expensive.
No firm will like a process that calls it out for excessive pricing.- Steve Morgan, health economist, University of British Columbia
As a result, Vertex is refusing to submit the newest version of its CF drug Symdeko to the health technology agencies in Canada, New Zealand and the U.K.
"The reason for not submitting Symdeko for review in Canada is that we anticipate that the same clinical criteria and cost effectiveness modelling approach that CADTH used for the review of Orkambi would also be used for Symdeko," a Vertex spokesperson told CBC News in an email. "This would lead to a similar outcome for Symdeko."
The company said it was not sending Symdeko to New Zealand's review agency either.
"We don't see a path forward for Symdeko in New Zealand; therefore, we have not submitted."
"No firm will like a process that calls it out for excessive pricing," said Morgan."This might be a sign of a changing strategy toward pointing at these health technology assessments as being flawed."
Without a review,CF patients in many countries will not get access to the new drug.
"That's to the detriment of patients who might have some benefit from that product," said Brian O'Rourke, CADTH's CEO. "We're open for business that if Vertex sends that submission into us we're perfectly willing to do a review as we would review any drug."
CEO gets million-dollar pay raise
Meanwhile, many of the world's CF patients are still waiting to get access toOrkambi while their governments are locked in price disputes with Vertex.

In the U.K., the fight has been messy. Vertex was accused in headlines of threatening the prime minister after the CEO sent an open letter referring to the company's investments in Britain.
In another incident, U.K. patients were furious when they learned that hundreds of doses of Orkambi were destroyed by Vertex because the drug expired while the company held out for a higher price.
"That's definitely a case where Vertex were choosing to hold off and try and squeeze out the highest possible price from the NHS [National Health Service, UK's public health agency] and would rather see drugs be destroyed and wasted rather than accepting a lower price and seeing those drugs getting to patients," said McDonald.
The whole drug dispute ended up in front of the U.K. parliament's health and social care committee where Vertex executives were questioned about the company's 40-per-cent growth in revenues and about the CEO's multi-million-dollar salary.
Last year, Vertex CEO Jeff Leiden was given a 9-per-cent raise. He now earns $18.8 million US, making him one of the highest paid executives in the biopharmaceutical industry.
"That's enough money to pay for hundreds of patients worth of treatment at the price that they're demanding from the U.K. government," said McDonald. "So I think there's a huge amount of anger that they're very, very willing to prioritize their own salaries and their own profits at the expense of children who need access to the drugs."

"Executive compensation is a very small part of our cost structure, which is overwhelmingly dedicated to R&D," Vertex told CBC News in an email.
McDonald's group has researcheda way to make a generic version of the drug for a fraction of the price ( 5,000 or $8,725Cdn per year). And it's urging the U.K. government to seize Vertex's patent on Orkambi which is permitted under a special provision of the law.
In France, there's another battle over the price of Orkambi. At one point, Vertex cancelled a series of French clinical trials,but backed down after a public outcry.
In Spain, patients wrote an angry letter to Vertex's CEO calling on the company to reach a price agreement with the Spanish government.
CF drugs based on Canadiandiscovery
Back in Canada, the lack of access to the first CF drugs that target theunderlying disease is especially poignant. That's because the drugs are based on a Canadian breakthrough by publicly funded scientists. In 1989, scientists at the Hospital for Sick Children in Torontomade medical history when they were part of a team that discovered the cystic fibrosis gene.
The drugs would not exist if not for that discovery and for the determined efforts of the Cystic Fibrosis Foundation, which raised millions of dollars to fund scientists at a biotechnology company to search for compounds that could correct the problems created by the defective gene.
The approach worked and it became clear that certain molecules could help reduce the mucus that gradually destroys the lungs of CF patients.
Vertex Pharmaceuticals took over the company and moved the CF drugs through the pipeline. In 2012, it launched the first of three CF treatments, each priced at more than a quarter of a million dollars a year.
At first a group of CF physicians protested the high prices, making this plea in aletter to Vertex's CEO:
"We write with the sincere hope that you will find a way to reflect the humility, generosity of spirit and consideration of the people not millionaires who will benefit from these drugs. Otherwise, we fear that they, and our medical care system, face ruin by costs driven by "what the market will bear."
But the price stayed high, and ever since, patient groups around the world have been lobbying governments for access to the most promising drugs for what isstill an incurable genetic disease.
To read the entire Second Opinion newsletter every Saturday morning,pleasesubscribe.












_(720p).jpg)


 OFFICIAL HD MUSIC VIDEO.jpg)
.jpg)



























































































