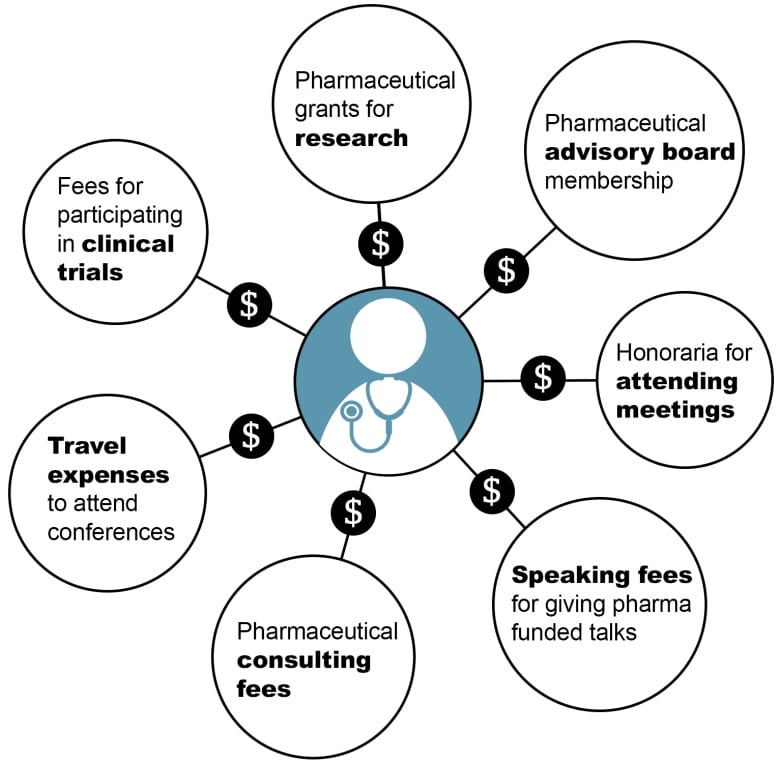
Does your doctor get money from drug companies? It's not easy to find out
New pressure for law to force disclosure of doctor-industry ties

By invitation only, 300 Canadian doctors have a chance to earn $6,600 on top of their usual public health-care fees by taking part in some research.
Here's what they have to do: sign up 12 high-risk heart patients, see each of them three times as part of normal practice, and fill out forms describing what drugs were prescribed.
The doctors get an additional $500 for attending a three-hour information session about the research. It's called the Guidelines Oriented Approach to Lipid Lowering (GOAL). And it is one example of how doctors receive money from outside the public system, often from the pharmaceutical industry.
GOAL is funded byAmgen, a pharmaceutical company. But it's being conducted by a private research organization called the Canadian Heart Research Centre, which is chaired byDr.AnatolyLanger. He designed the program and applied toAmgenfor funding.
GOAL's objective is to monitor whether doctors are following new guidelines when treating high-risk heart patients whose LDL cholesterol (so-called "bad" cholesterol) remains high despite taking commonly prescribed cholesterol-lowering drugs, calledstatins.
You could not get a committee in Canada with no conflicts of interest. Dr. Todd Anderson
If doctors follow the guidelines, they could put patients on additional drugs, potentially including a new one made by Amgen, called Repatha, which costs about $8,000 per year and is not covered by public drug plans.
Apart from the funding, Langer says Amgen has no further involvement in the program. The research is approved by independent ethics review boards, and the fees to the doctors are modest by industry standards, he says.
The company calls GOAL an "investigator initiated study" conducted by independent researchers.
"Amgen only supports these studies following a medical review to determine scientific merit," the company said in a statement to CBC News. It referred all questions to Langer.
Langer calls GOAL a "natural interest for all parties" because it reminds physicians to practise according to the guidelines, which are set by a committee of the Canadian Cardiovascular Society (CSS) to recommend best practices when it comes to lowering lipids.
Honoursystem
But a closer look at that committee is another example of the financial relationships that exist between Canada's doctors and the pharmaceutical industry.
Almost everyone on the 22-member committee received money from Amgen or other pharmaceutical companies.* Only two received no funding.
Yet according to the committee's own rules, no one with conflicts is allowed to vote on its recommendations.
So how did they manage the voting?The honour system. Each person made his or her own decision about whether they had a conflict of interest.
- THE FIFTH ESTATE |Doctors Without Boundaries
- ANALYSIS |Opioidconflict-of-interest controversy reveals extent of bigpharma'sties to doctors
"We declare the conflicts at the beginning and then ask them to recuse themselves if they have a real or perceived conflict of interest," said committee chairman Dr. Todd Anderson.
"It's just the fact of life in medicine," said Anderson. "You could not get a committee in Canada with no conflicts of interest. If you want the absolute experts who have been living and breathing this and know this work inside and out, they're going to have interactions with industry."
"Do I believe any of the members have a conflict because they've done a couple of talks for different companies? No I do not."

Across Canada, doctors are paid to sit on drug company advisory committees and to give industry-funded talks to other doctors. They also receive funding for research and are frequently asked to enrol patients in trials to test new drugs. The higher their profile, the more likely they are to receive industry funding.
"This relationship goes to the heart of the independence of the medical profession," said Prof. Matthew Herder, who researches health law ethics at Dalhousie University.
"Precisely because they have strong reputations and are considered important people within the field, and so they are identified by companies and they are paid to give a talk about products that the company is marketing."

Herder reviewed the industry relationships among leading cardiologists at Canadian universities and found many had received money for giving industry-sponsored talks to other doctors.
The issue made headlines recently, after Health Minister Jane Philpott ordered an independent review of new opioid guidelines because one member of that committee that voted on recommendations had received fees from Purdue Pharma and other companies that make opioid drugs, even though no one with any industry ties was supposed to vote.
- How doctors decide who gets a transplant
- THE FIFTH ESTATE | The High Cost of Pharmaceuticals
That spurred Dr. Andrew Boozary, a resident physician at St. Michael's Hospital in Toronto, and some colleagues to call for a law requiring industry payments to doctors be disclosed.
Right now there is no law in Canada forcing doctors or drug companies to disclose these relationships. There is no way for a patient to find out if their doctor is getting industry money, unless they ask.
"Not knowing the relationship between opioid manufacturers and prescribers, having that gaping blind spot as the health system tries to address the crisis, for us, just seemed to be unacceptable," Boozary said.
Voluntary disclosure
But in the U.S. if a doctor receives more than $10 from a drug company, it must be disclosed, by law, under the Physician Payments Sunshine Act, along with the details about why the payment was accepted. And it's searchable on a public database.
Tomorrow, ten of Canada's largest pharmaceutical companies will begin a form of voluntary disclosure of payments to doctors. They will post on their websites the amount they pay doctors in total for dinners, travel, speaking appearances and research.
But they will not be naming names or providing other individual details. And those ten companies represent only a fraction of Canada's pharmaceutical industry.
It's a move that falls short of the Sunshine Act.
"It is not sunshine, it's a complete fog," said Herder, at Dalhousie. " It's a P.R. move intended to give cover to these relationships which they know advantage companies' products.I would say it's a useless measure."
High-stakes decisions
But does simply disclosing payments eliminate the biases that could develop when doctors get money from drug companies?
A study published last month in the Journal of Clinical Oncology showed that U.S. oncologists receiving payments from particular companies prescribed that company's drug more often.
Conflicts of interest "may influence oncologists in high-stakes treatment decisions," the authors concluded.
Another study,published by theCochraneLibrary, found that studies sponsored by the manufacturer resulted in more favourable conclusions than studies sponsored by other sources.
Many other studies have found similar associations, but experts point out that associations do not necessarily prove that industry funding causes bias. The associations could also be explained by other factors, including the physician's increased familiarity with new drugs.
At the same time, some researchers have found evidence that pharmaceutical companies recruit doctors to be part of their clinical trials as a form of marketing, designing studies for the specific purpose of getting doctors familiar with using their new drugs. One study in 2016,published online atBioMedCentral, reported that "a fifth of drug trials published in the highest impact general medical journals in 2011 had features that were suggestive of being designed for marketing purposes."
Langer, at the Canadian Heart Research Centre, says he believesin disclosure. He says doctors should disclose the money he's paying them to be part of the GOAL study.
"I believe any payment including for participation in a clinical trial or a medical practice activity or in advisory boards or in train-the-trainer need to be disclosed," he said.
"Why not?If you're worried about disclosure you should probably be worried about participating in the exercise that you're worried about disclosing."
For Herder, at Dalhousie, the disclosure issue affects the entire health-care system.
"We need much more information about how money is changing hands," he said. "I think it's critical. It has implications for patient safety as well as health-care system sustainability."
* Sixteen of the 22 committee members received honoraria, consulting fees or clinical trial money from Amgen.Another four received money from other large pharmaceutical companies including Merck, AstraZeneca, Sanofi-Aventis, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Valeant, Takeda, Alexion, Eli Lilly, Pfizer, Novo Nordisk, Abott and GlaxoSmithKline.











_(720p).jpg)


 OFFICIAL HD MUSIC VIDEO.jpg)
.jpg)